A reagent kit for detection and quantification of mRNAs produced by chimeric ETV6-RUNX1 gene and reference ABL gene in clinical material by polymerase chain reaction (PCR) with real-time hybridization-fluorescence detection.
The reagent kit can be used to reveal AML associated with ETV6-RUNX1 chromosomal rearrangement, to confirm the AML diagnosis, and to monitor the efficiency of therapy, i.e., MRD evaluation.
The reagent kit
reagents is designed to conduct assays in a quantitative format for 24
clinical samples in duplicate (a total of 132 PCR reactions, including
controls).
Additional
information about the molecular marker
Chromosomal
translocation t(12;21)(p13;q22) produces a chimeric transcript ETV6 (TEL) - RUNX1 (CBFA2,
AML1). It is found in approximately 25%
of cases of childhood B-cell lymphoblastic leukemia, the most common variant of
acute lymphoblastic leukemia (ALL). The TEL and AML genes encode nuclear
transcription factors that play a critical role in normal hematopoiesis. Their
fusion leads to emergence and development of leukemia due to impaired TEL functioning,
and/or reduced expression level of the AML1 gene. There are two types of
TEL-AML translocation: the main variant occurs when the breakpoint of the AML gene is located in intron 1, and TEL gene, in intron 5. The other, less
common variant (10% of all TEL-AML cases), is formed by a break in the 2nd intron of AML1 and 5th intron of the TEL gene (see Fig. 1).
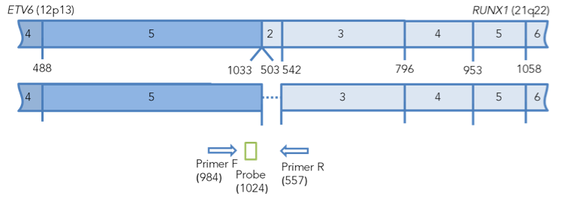
Figure 1. Arrangement of chimeric transcripts ETV6 (TEL) - RUNX1 (CBFA2, AML1). The binding sites of primers and probes for real-time PCR are marked. The positions of primers and probes are indicated relative to the 5' end of the nucleotide sequences of normal transcripts.
Accordingly, ETV6-RUNX1 translocation is an important target in the diagnosis and monitoring of minimal residual disease (MRD). When compared with standard methods (karyotyping, FISH), usage of real-time PCR (Real-Time Quantitative PCR, RQ-PCR) which measures ETV6-RUNX1 expression levels provides significantly higher sensitivity when assessing minimal residual disease (MRD), revealing one malignant cell among 50,000 normal ones.
More detailed information on diagnostic approaches, frequency of the study and prognostic value of this testing in leukemias are available on the website of the International Network European Leukemia Net (http://www.leukemia-net.org).
Ordering information:
ETV6-RUNX1 RQ Kit, 24 tests Cat.No IG-RQ-5-24
Additiional reagents:
Blood RNA stabilizer medium Cat.No IG-RSB-100
TriZ Reagent Kit Cat.No IG-TRK-100
ReverZyme Kit Cat.No IG-RT-1
Erythrocyte Lysis Solution Cat.No IG-TRL-100
Price: on request
Reagents are for research use only (RUO)